EFFECTIVNESS OF THERMAL IMAGING
The American Society of Breast Surgeons
Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer
Nimmi Arora, M.D., Diana Martins, B.S., Danielle Ruggerio, B.S., Eleni Tousimis, M.D., Alexander J. Swistel, M.D., Michael P. Osborne, M.D., Rache M. Simmons, M.D.*
Department of Surgery, New York Presbyterian Hospital–Cornell, New York, NY, USA
Abstract
BACKGROUND: Digital infrared thermal imaging (DITI) has resurfaced in this era of modernized computer technology. Its role in the detection of breast cancer is evaluated.
METHODS: In this prospective clinical trial, 92 patients for whom a breast biopsy was recommended based on prior mammogram or ultrasound underwent DITI. Three scores were generated: an overall risk score in the screening mode, a clinical score based on patient information, and a third assessment by artificial neural network.
RESULTS: Sixty of 94 biopsies were malignant and 34 were benign. DITI identified 58 of 60 malignancies, with 97% sensitivity, 44% specificity, and 82% negative predictive value depending on the mode used. Compared to an overall risk score of 0, a score of 3 or greater was significantly more likely to be associated with malignancy (30% vs 90%, P , .03).
CONCLUSION: DITI is a valuable adjunct to mammography and ultrasound, especially in women with dense breast parenchyma.
Digital infrared thermal imaging (DITI) is a noninvasive, non-contact system of recording body temperature by measuring infrared radiation emitted by the body surface. This technology was originally designed for US military use in night vision but also has many applications in medicine. Its use in the field of medical oncology lies in the fact that tumors generally have an increase in blood supply and angiogenesis, as well as an increased metabolic rate, which in turn translates into increased temperature gradients compared to surrounding normal tissue.1 Detecting these infrared “hotspots” and gradients can thereby help to identify and diagnose malignancy.
Infrared thermography has been in use in medical diagnostics since the 1960s, and in 1982 was approved by the US Food and Drug Administration (FDA) as an adjunctive tool for the diagnosis of breast cancer. Its applicability, however, was limited by the temperature resolution capability of earlier imaging technology, the bulky equipment necessary to perform procedures, and the general lack of computer analytical tools. Since then, major advances have been made in infrared thermal imaging technology, with digitalized high-resolution imaging and sophisticated artificial intelligence-based neural network image analysis. In the past, equipment for measuring infrared emission was only capable of resolving temperature variation from .5 to 1°C; some machinery required liquid nitrogen, and some even needed patient contact—a much more primitive technology requiring a special liquid crystal film to be placed on the patients’ breasts so as to detect temperature. The digital infrared thermography cameras of today are capable of sensing changes in temperature at .08°C or better and do not require any patient contact. Now, DITI has the capability of making significant impact in medicine.2 In this study we assess the effectiveness of a DITI system, the Sentinel BreastScan (SBS; Infrared Sciences Corp., Bohemia, NY USA), in detecting breast pathology in a group of patients with suspicious findings on either mammography or ultrasound that all underwent biopsy in a prospective, double-blinded trial.
Methods
Ninety-two women for whom a breast biopsy had been recommended on the basis of a previously suspicious mammogram or ultrasound were included in this 2-year study conducted at New York Presbyterian Hospital–Cornell. Informed consent was obtained from all patients and approval was obtained from our Institutional Review Board. Patients who were morbidly obese, had a bra size greater than DD, or had prior contralateral mastectomy were excluded due to technical limitations. The examination was performed with the patient disrobed from the waist up and positioned in a dedicated equipment suite with a chair equipped with lateral-view side mirrors, an integral air cooler, and a digital infrared camera. The digital camera was an uncooled focal plane array type with an image size of 320 x 240 pixels, sensitivity to .08°C, and an operating spectral (wavelength) range of 7–12 mm.
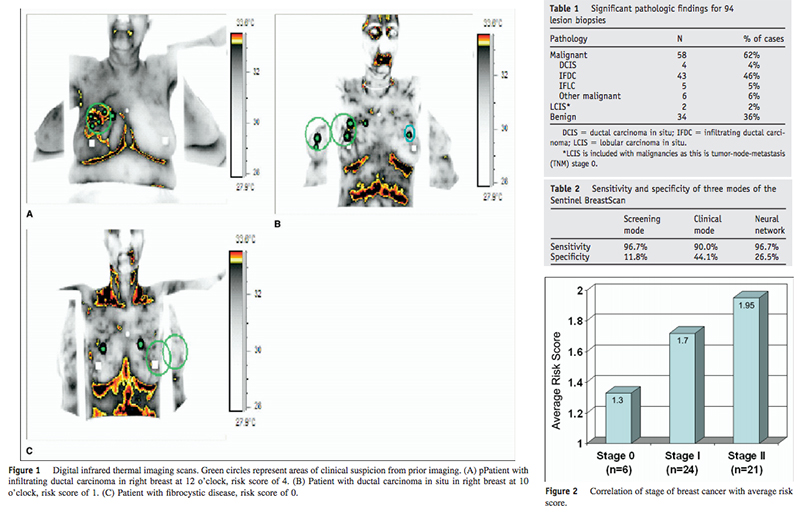
The examination took approximately 4 minutes per patient, where a dynamic series containing more than 100 temperature images was gathered during the administration of a cold stress (cool air directed at the breasts). The software extracted specific thermal parameters, performed asymmetry analysis between each breast, and focused on areas of the breasts that showed the greatest difference in temperature when compared with surrounding tissue. The program then produced a color-coded, processed image of the breasts showing suspicious foci, as well as results of all measured thermal breast parameters (Figure 1).
Each patient underwent 3 modes of analysis to generate 3 different scores. An overall risk score was tabulated in the blinded screening mode, giving a score of 0 (minimal risk) to 7 (very high risk). Any score greater than 0 was considered a positive (suspicious) finding. In the clinical mode, the location of the lesion under question based on prior imaging was assessed to generate a positive or negative clinical assessment. Finally, a third score was generated using an artificial neural network (ANN) evaluation to also give a positive or negative finding.
Statistical analysis was performed using Fischer exact test with P , .05 considered significant.
Results
The study consisted of 94 biopsies in 92 female patients with an average age of 51 years (range 23– 85). Of the 94 breast lesions, 60 were malignant (including 2 with lobular carcinoma in situ, since these tumors are considered stage 0) and 34 were benign on biopsy. As seen in Table 1, the majority of malignancies were infiltrating ductal carcinoma (IFDC). The median size of invasive tumors was 1.4 cm, with a range of .5–14 cm. Of 60 malignancies identified on biopsy, the SBS identified 58 correctly on both the screening mode and using ANN, and 54 of 60 using the clinical mode. Sensitivity and specificity for each of the modes of the SBS are given in Table 2. The negative predictive value for the SBS in this set of patients was 66.7% in the screening mode, 71.4% in the clinical mode, and 81.8% using ANN. All 4 ductal carcinoma in situ lesions were identified using the SBS system. Compared to an overall risk score of 0, a score of 3 or greater in the screening mode was significantly more likely to be associated with a cancer diagnosis (30% vs 90%, P , .03). Fifty-two of 59 patients with malignancy had surgically staged disease, either stage 0 (n 5 6), stage I (n 5 25), stage IIa (n 5 14), or stage IIb (n 5 7). There was a nonsignificant trend towards higher average risk scores for patients with malignancy at later stages of disease (Figure 2).
Conclusion
In this prospective clinical trial of 92 women undergoing DITI with suspicious breast lesions identified on prior mammogram or ultrasound, we have shown that the SBS can detect breast pathology with sensitivity up to 97% and a negative predictive value of 82%. DITI is painless, noninvasive, does not emit harmful radiation, has no patient risk, provides immediate results, and is relatively inexpensive. Compared to magnetic resonance imaging (MRI)—an adjunctive diagnostic tool for breast malignancy gaining more popularity—DITI is considerably more affordable to both patient and provider. MRI may cost $2,000 to the patient for Table 1 Significant pathologic findings for 94 lesion biopsies Pathology N % of cases Malignant 58 62% DCIS 4 4% IFDC 43 46% IFLC 5 5% Other malignant 6 6% LCIS* 2 2% Benign 34 36% DCIS 5 ductal carcinoma in situ; IFDC 5 infiltrating ductal carcinoma; LCIS 5 lobular carcinoma in situ. *LCIS is included with malignancies as this is tumor-node-metastasis (TNM) stage 0. Table 2 Sensitivity and specificity of three modes of the Sentinel BreastScan Screening mode Clinical mode Neural network Sensitivity 96.7% 90.0% 96.7% Specificity 11.8% 44.1% 26.5% Figure 2 Correlation of stage of breast cancer with average risk score. N. Arora et al. DITI and breast cancer 525 a range of .5–14 cm. Of 60 malignancies identified on biopsy, the SBS identified 58 correctly on both the screening mode and using ANN, and 54 of 60 using the clinical mode. Sensitivity or ultrasound, we have shown that the SBS can detect breast pathology with sensitivity up to 97% and a negative predictive value of 82%. DITI is painless, noninvasive, does not emit harmful radiation, has no patient risk, provides immediate results, and is relatively inexpensive.each examination and $2 million to own the equipment, while DITI costs less than $200 for each exam and approximately $25,000 to own the equipment.
The ability of DITI to detect tumors relies on the assumption that tumors have different biology from surrounding normal tissue. One study found a correlation between microvessel density of breast malignancies and thermographic hot spots, thus providing a mechanistic explanation for the use of DITI in cancer diagnosis.3 However, DITI is limited by the fact that thermal recordings are only a physiologic measure and therefore must be used as an adjunct to another test such as mammography or ultrasound. Infection or inflammation of breast parenchyma, for example, can also alter temperature recordings and lead to false positive findings. In addition, morbidly obese women and breast size greater than DD preclude accurate recording of temperature from the inferior aspect (undersurface) of the breasts, so these patients may not be ideal candidates for DITI. DITI is not currently recommended or approved as a substitute for screening mammography, and correlation of findings on DITI should be made with alternative imaging techniques.
One of the first studies to document the value of infrared thermography in the identification of breast cancer was by Gautherie and Gros in 1980.4 They reviewed thermograms performed on thousands of patients and found that patients with a “Thermogram stage Th IV or V” had a 90% chance of having cancer at time of study, and, more interestingly, 38% of 1,245 patients with Thermogram stage Th III (suspicious but not conclusive) developed cancer within 1– 4 years of follow-up. Other studies have since shown correlations of infrared thermography recordings with large breast tumor size, high grade, lymph node metastasis, and tumor vascularity.5,6 This is similar to our study where we showed a trend of higher risk scores correlating with higher stage of disease.
While previous thermography studies were limited by equipment, resolution, and sensitivity capabilities, the more sophisticated imaging and analytical tools available today make it is possible to use DITI and artificial neural networks to detect malignancy with up to 100% sensitivity.7 The low specificity of DITI in this particular pilot study is largely due to our select patient population, all with suspicious findings on prior radiologic examination. A separate population with nonsuspicious breast pathology will be needed to accurately assess the true specificity of DITI. Ultimately, evaluating a screening population with DITI will give clinicians and patients more information so as to determine who will necessitate a biopsy and who can be followed clinically in cases where mammography or ultrasound is inconclusive.
Patients who could potentially stand to benefit from this technology are those whose diagnosis of breast cancer can be difficult, including younger women, men, patients with dense breasts, or patients with surgically altered breasts (implants, breast reduction; provided nipples are intact for orientation and asymmetry analysis). Future studies using DITI for these individual groups can help to asses this potential.
In conclusion, we have shown that a modernized DITI system can be a useful adjunctive test in detecting breast cancer with 97% sensitivity in this prospective clinical trial of 92 patients.
References
1. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249 –57.
2. Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging 1998;17:1019 –27.
3. Yahara T, Koga T, Yoshida S, et al. Relationship between microvessel density and thermographic hot areas in breast cancer. Surg Today 2003;33:243– 8.
4. Gautherie M, Gros CM. Breast thermography and cancer risk prediction. Cancer 1980;45:51– 6.
5. Sterns EE, Zee B, SenGupta S, et al. Thermography. Its relation to pathologic characteristics, vascularity, proliferation rate, and survival of patients with invasive ductal carcinoma of the breast. Cancer 1996;77: 1324 – 8.
6. Head JF, Elliott RL. Thermography. Its relation to pathologic characteristics, vascularity, proliferation rate, and survival of patients with invasive ductal carcinoma of the breast. Cancer 1997;79:186 – 8.
7. Ng EY, Kee EC. Advanced integrated technique in breast cancer thermography. J Med Eng Technol, 2008;2:103–14.
Please help spread the word! Here are few ideas that can help raise awareness about breast health:
- Add information about Thermography to your newsletter
- Tweet about Thermography
- Add a Web badge to your Web site, blog, or social networking profile
- Talk about it to your friends
- Invite me to come and talk about Thermography to your church members, to your friends, to your employees
- Put this information on Facebook
